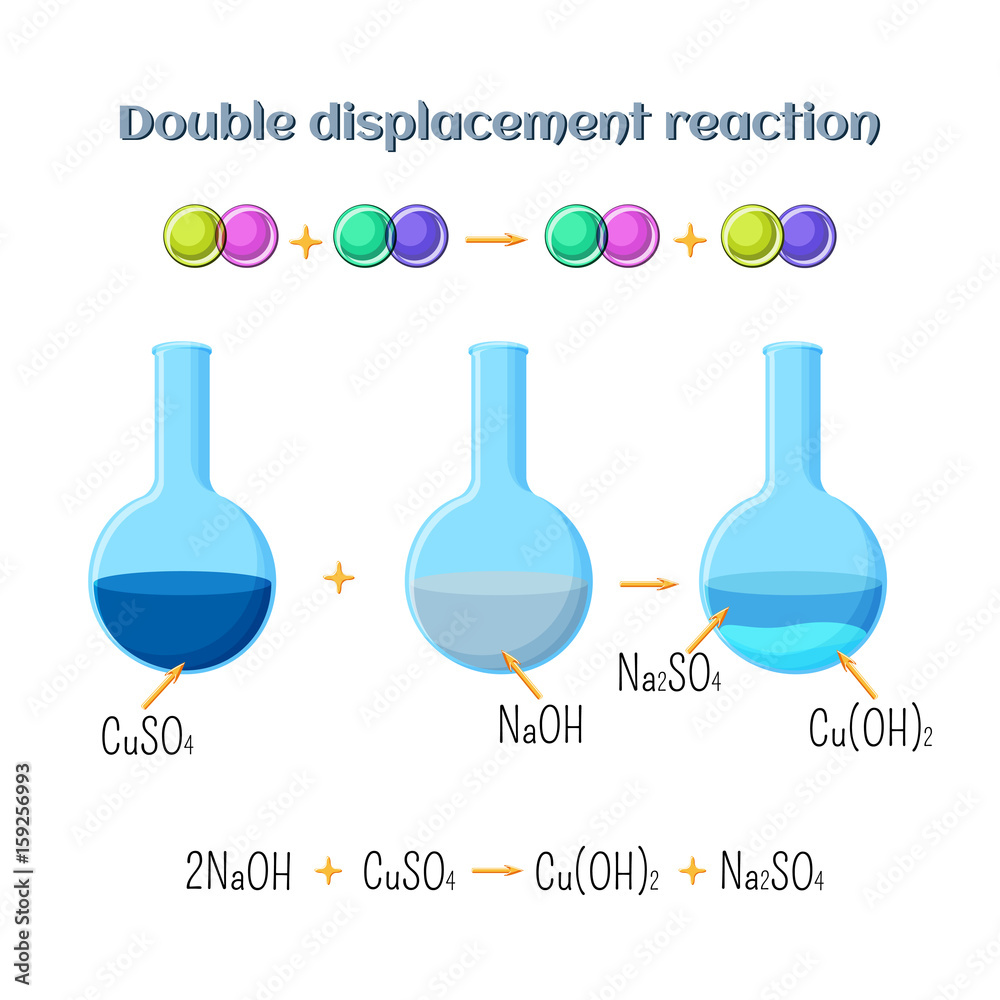
Copper Ii Sulfate Reacts With Sodium Hydroxide . i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. [5] for example, it reacts with iron to make copper and iron (ii). this video looks at the reactions of copper (ii) sulfate with a strong base (sodium. It reacts with most metals to make copper and a metal sulfate. it is a weak oxidizing agent. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. sodium hydroxide precipitates copper (ii) hydroxide: The result is a blue precipitate.
from stock.adobe.com
it is a weak oxidizing agent. It reacts with most metals to make copper and a metal sulfate. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. [5] for example, it reacts with iron to make copper and iron (ii). i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. The result is a blue precipitate. sodium hydroxide precipitates copper (ii) hydroxide: Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate.
Double displacement reaction sodium hydroxide and copper sulfate
Copper Ii Sulfate Reacts With Sodium Hydroxide [5] for example, it reacts with iron to make copper and iron (ii). two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. sodium hydroxide precipitates copper (ii) hydroxide: Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. it is a weak oxidizing agent. this video looks at the reactions of copper (ii) sulfate with a strong base (sodium. It reacts with most metals to make copper and a metal sulfate. The result is a blue precipitate. [5] for example, it reacts with iron to make copper and iron (ii).
From cristianmeowterry.blogspot.com
Molecular Equation for Copper Ii Sulfate and Sodium Phosphate Copper Ii Sulfate Reacts With Sodium Hydroxide in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. It reacts with most metals to make copper and a metal sulfate. it is a weak oxidizing agent. two moles of. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.doubtnut.com
Copper [II] sulphate solution. Reacts with sodium hydroxide solution to Copper Ii Sulfate Reacts With Sodium Hydroxide it is a weak oxidizing agent. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). It reacts. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Write the net ionic equation for the reaction of copper (II Copper Ii Sulfate Reacts With Sodium Hydroxide It reacts with most metals to make copper and a metal sulfate. this video looks at the reactions of copper (ii) sulfate with a strong base (sodium. The result is a blue precipitate. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. in this video we'll balance. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
Sodium Hydroxide and Copper Sulfate reaction YouTube Copper Ii Sulfate Reacts With Sodium Hydroxide two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. It reacts with most metals to make copper and a metal. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper Ii Sulfate Reacts With Sodium Hydroxide two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. it is a weak oxidizing agent. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
Amino Acids 3. Reactions with sodium hydroxide, sodium carbonate Copper Ii Sulfate Reacts With Sodium Hydroxide sodium hydroxide precipitates copper (ii) hydroxide: here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). [5] for example, it reacts with iron to make copper and iron (ii). i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. Cu + 2 (aq) + 2oh. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
Sodium hydroxide and ammonia added to copper (II) chloride solution Copper Ii Sulfate Reacts With Sodium Hydroxide this video looks at the reactions of copper (ii) sulfate with a strong base (sodium. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the following equation:. it is a weak oxidizing agent. two moles. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Tetraamminecopper(II) Sulfate The first step in the synthesis Copper Ii Sulfate Reacts With Sodium Hydroxide this video looks at the reactions of copper (ii) sulfate with a strong base (sodium. sodium hydroxide precipitates copper (ii) hydroxide: use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. i tried reacting copper sulfate with sodium hydroxide to get copper hydroxide, which should precipitate, according to the. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.coursehero.com
[Solved] Pls help. 4. Precipitation reactions. The reaction of copper Copper Ii Sulfate Reacts With Sodium Hydroxide It reacts with most metals to make copper and a metal sulfate. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. The result is a blue precipitate. sodium hydroxide precipitates copper (ii). Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
Reaction of Copper Sulphate (CuSO4) with Sodium Hydroxide (NaOH) YouTube Copper Ii Sulfate Reacts With Sodium Hydroxide It reacts with most metals to make copper and a metal sulfate. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. this video looks at the reactions of copper (ii). Copper Ii Sulfate Reacts With Sodium Hydroxide.
From askfilo.com
1. Copper (II) sulphate solution reacts with sodium hydroxide solution to.. Copper Ii Sulfate Reacts With Sodium Hydroxide two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. sodium hydroxide precipitates copper (ii) hydroxide: It reacts with most metals to make copper and a metal sulfate. this video looks at the reactions of copper (ii) sulfate with a strong base (sodium. i tried reacting copper. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Question 2 (5 points) Copper(II) sulfate, CuSO4, reacts with Copper Ii Sulfate Reacts With Sodium Hydroxide here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). It reacts with most metals to make copper and a metal sulfate. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. sodium hydroxide precipitates copper (ii) hydroxide: [5] for example, it reacts with iron to make copper. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From stock.adobe.com
Interaction of solutions of sodium hydroxide and copper II sulfate. The Copper Ii Sulfate Reacts With Sodium Hydroxide use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. [5] for example, it reacts with iron to make copper and iron (ii). Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. The result is a blue precipitate. it is a weak oxidizing agent.. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii Sulfate Reacts With Sodium Hydroxide this video looks at the reactions of copper (ii) sulfate with a strong base (sodium. [5] for example, it reacts with iron to make copper and iron (ii). two moles of aqueous sodium hydroxide plus one mole of aqueous copper (ii) sulfate produces one mole of aqueous. sodium hydroxide precipitates copper (ii) hydroxide: it is a. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From brainly.in
Copper sulphate reacts with sodium hydroxide to form blue precipitate Copper Ii Sulfate Reacts With Sodium Hydroxide [5] for example, it reacts with iron to make copper and iron (ii). in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. it is a weak oxidizing agent. sodium hydroxide precipitates copper (ii) hydroxide: The result is a blue precipitate. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From dxoegbjnx.blob.core.windows.net
Copper Ii Sulfate Sodium Phosphate Ionic Equation at Marie Michell blog Copper Ii Sulfate Reacts With Sodium Hydroxide it is a weak oxidizing agent. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. this video looks at the. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper Ii Sulfate Reacts With Sodium Hydroxide here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). in this video we'll balance the equation cuso4 + naoh = cu (oh)2 + na2so4. Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. The result is a blue precipitate. two moles of aqueous sodium hydroxide plus one. Copper Ii Sulfate Reacts With Sodium Hydroxide.
From exotjinqn.blob.core.windows.net
Copper Ii Sulfate Chemical Formula at Jack Butler blog Copper Ii Sulfate Reacts With Sodium Hydroxide sodium hydroxide precipitates copper (ii) hydroxide: Cu + 2 (aq) + 2oh − (aq) − ⇀ ↽ − cu(oh) 2(s) the precipitate. this video looks at the reactions of copper (ii) sulfate with a strong base (sodium. here, copper (ii) sulfate (cuso 4) is added to sodium hydroxide (naoh). i tried reacting copper sulfate with sodium. Copper Ii Sulfate Reacts With Sodium Hydroxide.